Abstract
Introduction
In recent decades, many novel anticancer therapies have been developed resulting in improved patient outcomes across numerous tumor types - including lymphoid malignancies. However, new treatments and treatment combinations have also introduced new and sometimes serious toxicities. Transparent, objective, and complete toxicity reporting is critical to ensuring patient-centered, evidence-informed care. Unfortunately, subjective terms such as "tolerable” or "acceptable toxicity” are widely used and may normalize high rates of toxicity (Gyawali et al. BMJ 2018). The patterns of toxicity reporting and subjective terminology use in clinical trials presented at major conferences such as ASH, where practice changing data is often first shared, remains largely unknown. We conducted a systematic review of lymphoma and myeloma abstracts presented at ASH annual meetings from 2017-2021 to determine patterns of toxicity reporting and to measure the use of subjective, minimizing language in phase 3 clinical trials.
Methods
Following an initial pilot phase to characterize common subjective, minimizing language terms, we performed a systematic review of abstracts presented at ASH between 2017 - 2021. We included abstracts of adult or pediatric, phase 3, randomized control trials (RCTs) in lymphoma and multiple myeloma (MM). Abstracts reporting on subset analyses of a larger trial, long-term follow up, supportive care interventions, and non-pharmacological interventions were ineligible. Publication year, study population, treatment, AE/toxicity reporting, and minimizing language were extracted and analyzed. Subjective, minimizing language was defined as the use of: "acceptable", "tolerable", "manageable", "feasible", "favorable", "limited", and "safe".
Results
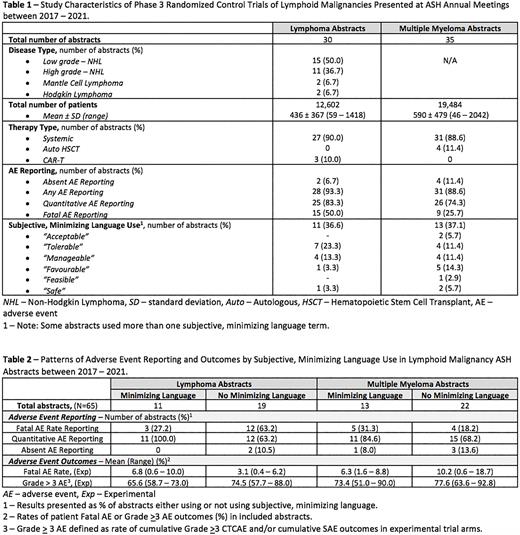
902 abstracts were reviewed, of which 65 met criteria for inclusion (30 lymphoma and 35 myeloma). The characteristics of eligible studies are presented in Table 1. A majority of abstracts reported on systemic therapies, 3 reported on CAR-T (all in lymphoma), and 4 on autologous stem cell transplant, (all in MM).
AE reporting was variable. Six abstracts did not provide any information on AE (2 lymphoma and 4 MM). Notably, only 15 (50%) of the lymphoma abstracts and 9 (26%) of the MM abstracts provided information on the occurrence or absence of fatal adverse events. Most abstracts included quantitative data on AE (83% of lymphoma abstracts and 74% of MM): however, the toxicity reporting was not uniform with some trials reporting cumulative and/or specific Common Terminology Criteria for Adverse Events (CTCAE)s, others citing only specific toxicities (e.g., cytopenia), and others reporting only "treatment related” and/or "treatment emergent” AE. Encouragingly, all 3 CAR-T trials presented standardized, quantitative data on cytokine release syndrome (CRS) and neurotoxicity AE.
Subjective, minimizing language was used in 37% of abstracts (Table 2). Among those abstracts using subjective minimizing language, 33% provided information on fatal AE rates (Table 2). The rates of fatal AE in the experimental arms ranged from 0.6 - 10%. The rates of > grade 3/SAE experimental arm toxicities in abstracts that used subjective language ranged from 58.7 - 90%. Data on reporting of quality of life, patient reported outcomes, and treatment discontinuation will be presented.
Discussion
Our systematic review of phase 3, lymphoma and MM RCTs presented at recent ASH Annual Meetings suggests that the use of subjective, minimizing language to describe drug toxicity is common. Moreover, trials using subjective, minimizing language reported relatively high rates of serious, and even fatal, AE. In addition, reporting of AE outcomes was inconsistent and variable across both disease types. Patient-centered, evidence-informed care requires transparent, objective, and complete reporting of benefits and risks of new therapies- including in abstracts which can be practice changing. Implementation of policies to standardize AE reporting, and to discourage use of subjective, minimizing language in conference abstracts should be considered.
^ - co-first authors; # - co-senior authors
Disclosures
Lyman:G1 Therapeutics: Consultancy; Samsung Bioepis: Consultancy; Merck: Consultancy; Squibb: Consultancy; Sandoz: Consultancy; Seattle Genetics: Consultancy; Fresenius Kabi: Consultancy; Beyond Spring: Consultancy; Amgen: Research Funding. Kuderer:Beyond Spring: Consultancy; Bristol Myers Squibb: Consultancy; G1 Therapeutics: Consultancy; Sandoz: Consultancy; Seattle Genetics: Consultancy; Janssen: Consultancy; Pfizer: Consultancy; Spectrum: Consultancy; Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal